Pipeline
Innovation Platform
 Specialized Brain-Penetrant Drug Development Platform
Specialized Brain-Penetrant Drug Development PlatformAs population aging accelerates, the prevalence of brain diseases continues to rise, creating an urgent and growing need for effective therapies. Yet the brain, our central command system, presents extraordinary challenges for drug development due to its highly complex structure. The blood–brain barrier (BBB), a unique protective interface, prevents more than 98% of drug molecules from entering the brain. Many promising drug candidates fail for this reason alone. Overcoming the BBB and achieving efficient brain penetration has therefore become the critical bottleneck in developing treatments for neurological disorders.
Hyperway Pharma has built a specialized, high‑efficiency platform dedicated to the development and evaluation of brain‑penetrant drugs. Supported by a professional R&D team, strong technical capabilities, and advanced precision equipment, the platform integrates molecular design, synthesis and screening, pharmacokinetic assessment, and toxicology research. Its mission is to consolidate R&D resources, strengthen collaborative innovation, and accelerate the development of highly brain‑penetrant therapeutics that can benefit patients worldwide.
To date, the platform has established multiple innovative pipelines spanning primary and secondary brain tumors, autoimmune diseases, and pain. Several neurological drug candidates have demonstrated brain penetration rates exceeding 60%. Among them, HBW‑3210 capsules have successfully advanced into Phase I clinical trials.

 Differentiated R & D pipeline
Differentiated R & D pipelineHomogeneous competition remains one of the major challenges facing innovative drug development in China. Hyperway Pharma has remained committed to building a distinctive corporate identity by focusing on high‑value disease areas such as pain management and oncology. In addition to establishing a specialized brain‑penetrant drug development platform dedicated to creating novel therapeutics capable of crossing the blood–brain barrier, the company rigorously selects validated, cutting‑edge targets and pursues the development of Best‑in‑Class (BIC) and First‑in‑Class (FIC) candidates. By leveraging first‑mover advantages to secure early positions in emerging therapeutic fields, Hyperway Pharma strengthens its competitive edge and advances its long‑term market leadership. This differentiated strategy not only enables the company to stand out in a crowded landscape but also steadily reinforces its reputation as an industry pacesetter.

 Professional R&D Expertise
Professional R&D ExpertiseInnovative drug development is a highly knowledge‑intensive scientific endeavor. Hyperway Pharma has established a professional core R&D team with international experience and forward‑looking vision, supported by a strong mid‑level backbone primarily composed of post‑80s talent. Together, the team spans the entire lifecycle of innovative drug development—from target identification and preclinical research to clinical development, regulatory registration, and commercialization.
This integrated structure enables Hyperway Pharma to cultivate distinctive strengths in pipeline strategy and planning, scientifically rigorous and innovative drug design, advanced and efficient complex‑synthesis technologies, highly competitive CMC capabilities, and rapid, high‑quality execution throughout clinical development. These capabilities form the foundation of the company's differentiated innovation engine and long‑term competitiveness.

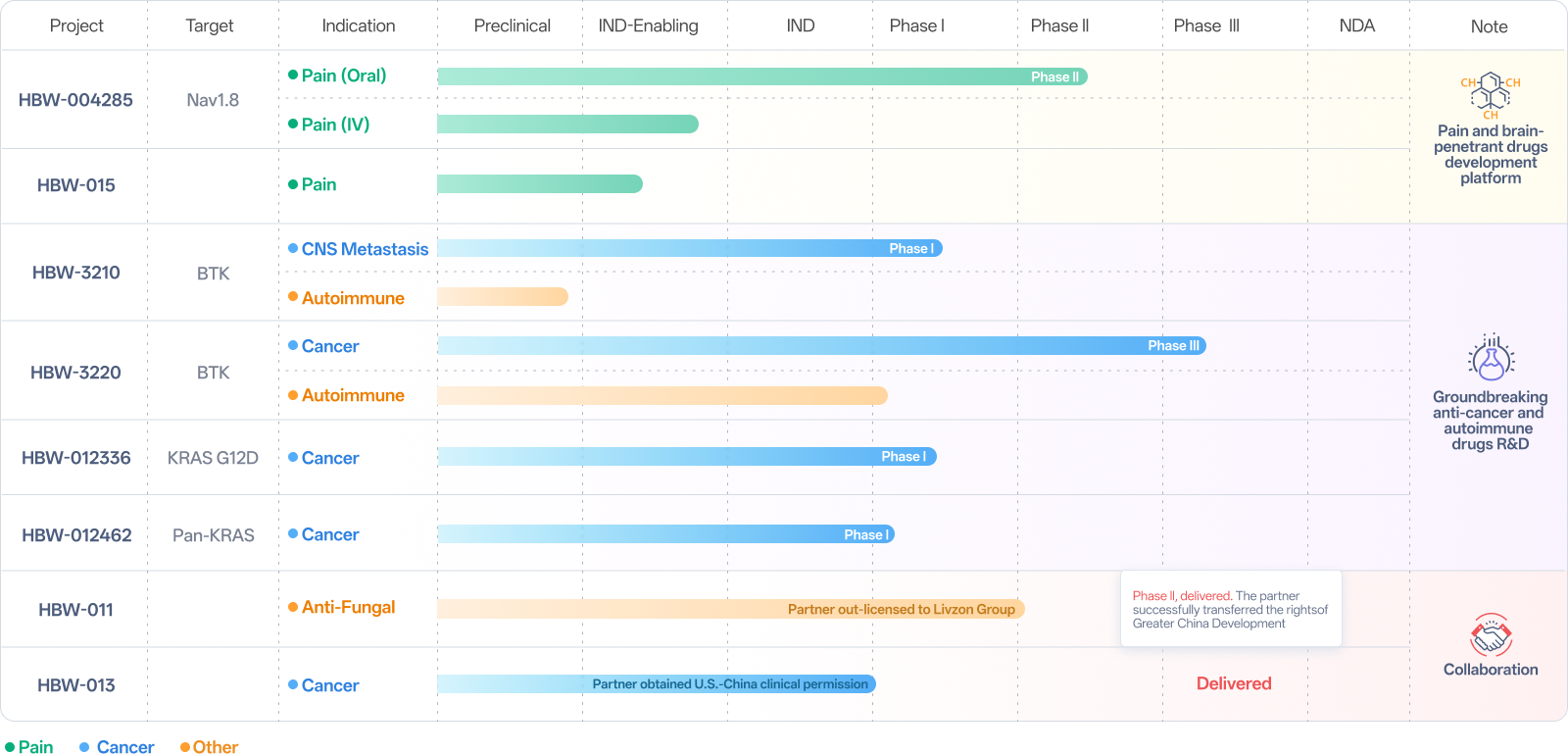
Pipeline Flagship Under Development
The analgesic drug market presents a vast unmet demand. According to data from Zhiyan Consulting (Intelligence Research Group), the global analgesic drug market size reached approximately $91.14 billion in 2022, while China's analgesic drug market size amounted to about 122.6 billion yuan. Hyperway Pharma' novel mechanism analgesic HBW-004285 tablets, a Nav1.8 inhibitor, does not produce opioid-like addictive properties and is expected to emerge as one of the game-changers in the traditional analgesics market.
HBW-004285 tablets have entered Phase II clinical trials. Phase I clinical data indicates that compared to VX-548—the world's first approved Nav1.8 inhibitor—HBW-004285 demonstrates superior safety, faster onset of action, optimized pharmacokinetics, and no gender-related differences, positioning it to potentially outperform VX-548.
Bruton's tyrosine kinase (BTK) is a key regulator of B cell receptor signal transduction pathway, which is closely related to a variety of B cell tumors (such as lymphoma, leukemia)
and autoimmune diseases (such as systemic lupus erythematosus, multiple sclerosis). Although only six BTK inhibitors have been approved to market in the world, they have achieved great success in the treatment of lymphoma and leukemia, giving rise to the blockbuster drug ibrutinib with annual sales exceeding $9 billion. And with the application of BTK inhibitors in the field of autoimmune diseases, it is expected that the global market size of BTK inhibitors will reach $26.1 billion by 2030. However, the resistance of the first and second generation BTK inhibitors has inevitably emerged, so scientists have developed non covalent BTK inhibitors that do not rely on the C481 site.
HBW-3220 capsules are poised to initiate Phase III clinical trials. As the world's most rapidly advancing fourth-generation BTK inhibitor, it overcomes multiple drug-resistant mutations (such as C481S, L528W, T474I) developed against the first three generations of BTK inhibitors. Available research data demonstrate HBW-3220's favorable safety profile and significant therapeutic efficacy. It exhibits particularly outstanding results in patients with chronic lymphocytic leukemia (CLL) and small lymphocytic lymphoma (SLL), achieving an objective response rate (ORR) of 100% in CLL/SLL patients harboring the C481S mutation. For CLL/SLL patients who have previously taken irreversible BTK inhibitors and BCL2 inhibitors, its ORR has surpassed Pirtobrutinib, positioning it to potentially overtake competitors and capture the follow-on treatment market for BTK inhibitors valued at tens of billions of dollars.
Additionally, HBW-3220 capsules hold significant development potential in the field of autoimmune diseases, having already received a clinical trial approval for an expanded indication in nephropathy.
KRAS was once considered an “undruggable target.” The KRAS G12D mutation is the most common type of KRAS mutation and occurs more frequently in cancers that are difficult to treat or have poor treatment outcomes, such as pancreatic cancer. Studies indicate that approximately 90% of pancreatic cancer patients harbor KRAS mutations, with KRAS G12D mutations accounting for the highest proportion at 45% (compared to only 1% for G12C mutations). Furthermore, in colorectal cancer—the third most common cancer globally—the G12D mutation accounts for 45% of KRAS-mutated cases. Consequently, developing KRAS G12D inhibitors not only holds significant market potential but also addresses an urgent need to safeguard the health and lives of millions of patients.
Leveraging the capabilities in drug design, synthesis, and comprehensive drugability evaluation, Hyperway Pharma has developed HBW-012336—an orally administered KRAS G12D inhibitor exhibiting high activity, selectivity, tissue targeting, and potent antitumor efficacy. This project received clinical trial approval in April 2025 and is currently undergoing Phase I clinical trials.
KRAS mutations are diverse, encompassing not only G12C and G12D mutations but also over 20 other variants such as G12V, G12R, and G13D. Among these, the seven predominant mutations account for more than 90% of all KRAS mutations. Therefore, developing Pan-KRAS inhibitors capable of broadly suppressing the core spectrum of KRAS mutations has become a hotspot in current drug research and development, with the potential to become one of the most powerful anti-cancer weapons in the future. Estimates suggest that KRAS G12C and KRAS G12D inhibitors alone will constitute a market worth approximately $10 billion. Pan-KRAS inhibitors, however, will overcome the limitations of single-mutation KRAS inhibitors by covering multiple mutation types, overcoming resistance, and offering broader applicability and superior combination effects. Their market value is projected to be eight times that of KRAS G12C inhibitors, attracting numerous pharmaceutical companies to enter the field.
Hyperway Pharma' high-potency, highly selective, and tissue-targeted oral Pan-KRAS inhibitor HBW-012462 simultaneously inhibits both KRAS (ON) and KRAS (OFF) states. Compared to publicly available data on Pan-KRAS inhibitors currently in development globally, HBW-012462 demonstrates superior or comparable performance in key preclinical drug-development metrics including cellular activity, in vivo efficacy, and pharmacokinetics, indicating significant development potential. Clinical studies are currently underway.
The significant increase in the types and number of patients with neurological disorders has created an increasingly urgent global demand for neurological drugs, driving rapid growth in the pharmaceutical market. This market is projected to reach $172.1 billion by 2034. Hyperway Pharma has established a brain-penetrant drug development platform with distinct competitive advantages, focusing on developing brain disease drugs capable of penetrating the blood-brain barrier. The company has deployed multiple innovative drug pipelines and identified several brain-targeted drugs with brain penetration rate exceeding 60%. HBW-3210 Capsule is one such promising candidate.
HBW-3210 Capsule features two major innovations: First, as a fourth-generation non-covalent BTK inhibitor, it overcomes resistance issues caused by the C481S mutation in first and second generation BTK inhibitors, demonstrating high inhibitory activity against both wild-type and mutant BTK kinases. Second, it exhibits potent brain penetration. Compared to Tirabrutinib (developed by Ono Pharmaceutical in Japan)—the world's only approved first-generation irreversible BTK inhibitor for relapsed or refractory PCNSL—HBW-3210 demonstrates superior brain permeability (rat BBB penetration rate: >60% vs ~10%) and efficacy. This demonstrates significant development potential and the possibility of becoming a FIC compound.
Currently, HBW-3210 capsules are undergoing Phase I clinical trials and have demonstrated remarkable therapeutic effects. Notably, a patient who progressed due to resistance to a previously used brain-penetrant BTK inhibitor achieved complete disappearance of brain tumors after six cycles of HBW-3210 capsules, achieving outstanding therapeutic outcomes.
Collaboration
-
 Innovative Medicines
Innovative Medicines
Regional Rights Assignment -
 Innovative Medicines
Innovative Medicines
Collaborative Development -
 Innovative drug
Innovative drug
R&D technology transfer -
 Other Medicinal Products
Other Medicinal Products
Research and Development Collaboration
Innovative drug development is a lengthy and challenging process. As China's pharmaceutical industry undergoes a period of profound transformation, Hyperway Pharma will adopt an open, multi-tiered, and multi-faceted collaboration model to maximize resource integration, concentrate R&D efforts, accelerate drug development, and avoid homogenized competition. Embracing an attitude of “The sea admits hundreds of rivers,” we seek like-minded partners both domestically and internationally to jointly develop high-quality, cost-effective Chinese medicines!