About Hyperway
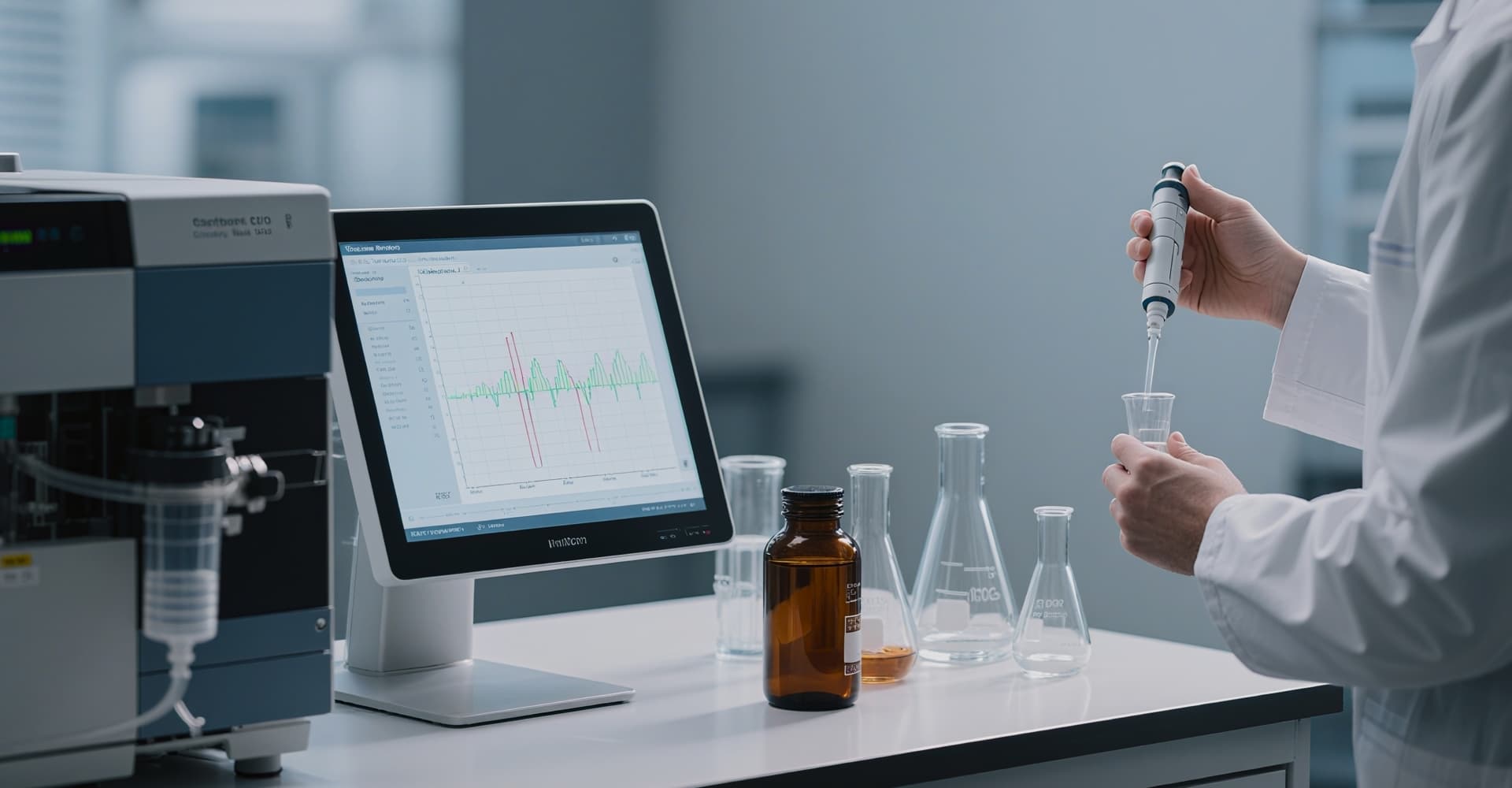
Guided by its corporate mission of “Develop Quality Medicines, Innovate in China,” Hyperway Pharma has built an international, highly specialized core R&D team equipped with forward‑looking strategic vision and robust innovation resources. Anchored in unmet clinical needs and driven by scientific advancement, the company is dedicated to developing First‑in‑Class (FIC) and Best‑in‑Class (BIC) targeted therapies. Hyperway Pharma is committed to becoming a globally influential biopharmaceutical innovator, continuously contributing to the improvement of human health.

Founded in
2019

R&D Pipeline
15
+

Patent Application
70
+
Clinical Trials
5

Pipeline Flagship Under Development
Hyperway News